Seawater desalination by solar evaporation
Electrostatic repulsion for electricity generation
Solar desalination presents an exciting opportunity to simultaneously produce fresh water and generate electricity using sustainable methods. During my PhD research, I explored the use of alginate-based composites, crosslinked with multivalent cations like aluminum and zirconium, to recover lithium from seawater. These materials exhibited a surprising ability to repel dissolved cations—an effect that led me to ask a new question: Could the repulsive ionic forces within the alginate matrix create an internal salinity gradient strong enough to generate electricity during solar evaporation? This post introduces the scientific background behind that idea, which merges seawater desalination, ion-selective membranes, and solar energy into a unified system. Further details on lithium recovery applications will follow in a future post.
Electricity is generated through the creation of an electrical potential difference. In the context of solar desalination and evaporation-driven systems, electricity can be harvested when electrolytes, such as seawater, move through a nanoporous membrane or charged microchannel. For efficient energy conversion, the size of these channels must be close to the Debye length of the fluid—allowing the formation of an electrical double layer (EDL) by counterions responding to the negatively charged surface.
As evaporation-induced hydrodynamic pressure drives fluid through the nanopores, the mobile counterions are carried downstream. This movement of charge generates a potential difference known as the streaming potential, which can be harnessed for power generation. For a more in-depth explanation of this mechanism, refer to the work of Dr. Jun Hong Park, who conducted a parallel study on evaporation-induced energy harvesting from seawater.
Salinty gradient generated within alginate composites
In contrast, alginate composites crosslinked with multivalent cations inherently exhibit ion-repulsive properties toward seawater cations. During the evaporation process, water molecules migrate to the upper surface and undergo phase transition, while the transport of cations is suppressed due to electrostatic interactions with the crosslinked matrix. This mechanism results in the establishment of a vertical salinity gradient, characterized by a lower ionic concentration near the evaporation surface compared to the base. The resultant ionic disequilibrium gives rise to an electrical potential difference. The magnitude and configuration of this gradient are influenced by the specific type of crosslinking cation employed, as illustrated in the schematic below.
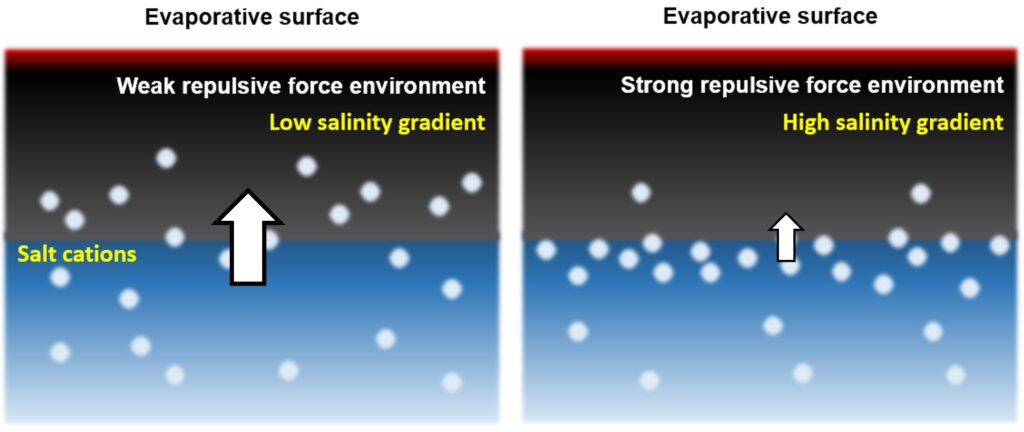
Based on this concept, I tested the electricity generation performance using tri-positive (3+) aluminum and tetra-positive (4+) zirconium crosslinked alginate composites. As expected, the zirconium-crosslinked alginate composite generates electricity more stably compared to the aluminum-crosslinked alginate. This result leads us to believe that we no longer need to create nanopores to generate electricity; instead, we can do so through a simpler method just by crosslinking alginate with these cations and incorporating polypyrrole, a light-absorbing and electrically conducting polymer.
While we generate electricity, we can also easily obtain fresh water during the solar evaporation process at the same time. I believe this novel phenomenon has the potential to advance our field of desalination. However, despite the simplicity of the method, the efficiency needs improvement. This study focuses to verify the feasibility of simultaneous generation of electricity and fresh water (and I did). So, those materials just had an evaporation rate of 1.15 kg/h/m2 and a conversion efficiency of 54.12% under the 1 sun solar irradiation condition. The efficiency varies depending on the types of incorporated polymers and the structures of the polymer networks, where a trade-off may exist between electricity generation and fresh water production.
Numerous studies have demonstrated the potential of solar evaporation systems for efficient electricity generation and water production. Building upon these foundational findings, future research could focus on optimizing polymer compositions and network architectures to maximize performance. Addressing these challenges may pave the way for more sustainable and cost-effective approaches to seawater desalination and renewable electricity generation, with significant implications for resource-limited communities worldwide.
This study was published in the journal of Desalination. For the details of this study, click Here (link).

